Plastics
Bend me, shape me, anyway you want me. Those are the words of an old love song, but it could just as easily be a song about plastics—the most versatile materials in our modern world. Plastics are plastic, which means we can mold them into pretty much anything, from car bodies and washing-up bowls to toilet seats and toothbrushes. That’s partly because there are many different kinds of plastic but also because each kind can be used for many things. What exactly is plastic? How do we make it? How do we get rid of it when we no longer need it? Let’s take a closer look!
What are plastics?
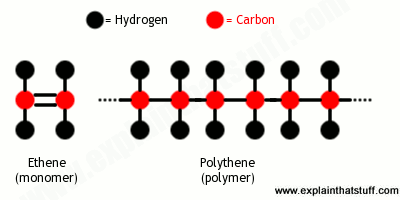
We talk about “plastic” as though it’s a single material, but there are in fact many different plastics. What they have in common is that they’re plastic, which means they are soft and easy to turn into many different forms during manufacture. Plastics are (mostly) synthetic (human-made) materials, made from polymers, which are long molecules built around chains of carbon atoms, typically with hydrogen, oxygen, sulfur, and nitrogen filling in the spaces. You can think of a polymer as a big molecule made by repeating a small bit called a monomer over and over again; “poly” means many, so “polymer” is simply short for “many monomers.” If you think of how a long coal train is made from many trucks coupled together, that’s what polymers are like. The trucks are the monomers and the entire train, made from lots of identical trucks, is the polymer. Where a coal train might have a couple of dozen trucks, a polymer could be built from hundreds or even thousands of monomers. In other words, polymers typically have very large and heavy molecules.
Types of plastics
 There are many different plastics, so we need ways of making sense of them all by grouping similar ones together. Here are a few ways we can do that (and there are others I’ve not listed):
There are many different plastics, so we need ways of making sense of them all by grouping similar ones together. Here are a few ways we can do that (and there are others I’ve not listed):
- We can split them into natural (ones easily obtained from plants and animals) and synthetic (ones artificially made by complex chemical processes in a factory or lab). Cellulose is a natural polymer used for making sticky tape (among other things), whereas nylon is a synthetic polymer made in a factory.
- We can group them according to the structure of the monomers that their polymers are made from. That’s why we talk about polyesters, polyethenes, polyurethanes and so on—because they’re different polymers made by repeating different monomers.
- When it comes to recycling, we need to separate plastics into different kinds that can be processed together without causing contamination. That depends on their chemical properties, physical properties, and the polymer types from which they’re made, and gives us seven main kinds. (You’ve probably noticed seven different recycling symbols numbered 1-6 and “null” on plastic packaging, if you’ve looked carefully.)
- We can group by what they’re made from (say bioplastics—artificially made from natural ingredients) or how they behave when they’re buried in landfills (biodegradable, photodegradable, and so on).
- We can split them into two broad kinds according to how they behave when they’re heated: thermoplastics (which soften when they’re heated) and thermosets (thermosetting plastics, which never soften after they’re initially molded).
Thermoplastics and thermosets
The last one on my list is such an important way of grouping plastics that we’d better look at it in a bit more detail. What’s the difference between thermoplastics and thermosets—and how can we explain it?
Thermoplastics

You can make something like a plastic bottle by injecting hot, molten plastic into a mold, then letting it cool down. Your bottle stays solid, but if you heat it up again later, it’ll soften and melt. We say it’s made from a thermoplastic: something that becomes plastic (soft and flexible) when it meets thermal energy (heat). In a thermoplastic, the long polymer molecules are joined to one another by very weak bonds, which easily break apart when we heat them, and quickly reform again when we take the heat away. That’s why thermoplastics are easy to melt down and recycle. Some everyday examples you will have come across are polyethylene/polythene (plastic bottles and sheets), polystyrene (crumbly white packaging material), polypropylene (plastic ropes), polyvinylchloride/PVC (toys and credit cards), polycarbonate (hard plastic windows and car headlamps), and polyamide (nylon—used in everything from stockings and swimming shorts to toothbrushes and umbrellas).
Thermosetting plastics (thermosets)

Thermosets are usually made from much much bigger polymer chains than thermoplastics. When they’re initially manufactured, they’re heated or compressed to form a dense, hard, structure with strong cross-links binding each of these long molecular chains to its neighbors. That’s very different from thermoplastics, where the polymer chains are held to one another only by very weak bonds. And that’s why we can’t simply heat thermosets to remold or reform them. Once they’re “set” (cured) during manufacture, they stay that way. You’ll be less familiar with thermosets than with thermoplastics; even so, you may have come across examples like polyurethane (insulating material in buildings), polytetrafluoroethylene/PTFE (nonstick coatings on cooking pots and pans), melamine (hard plastic crockery), and epoxy resin (a tough plastic used in strong adhesives and wood fillers).
How do we make plastics?
We’ve already seen that plastics are made from polymers, but how are polymers made? They’re based on hydrocarbons (molecules built from hydrogen and carbon atoms) that we get mostly from things like petroleum, natural gas, or coal. Crude oil drilled from the land or sea is a thick gloopy mixture that contains thousands of different hydrocarbons, which have to be separated out before we can use them. That happens in an oil refinery, through a process called fractional distillation. It’s a more involved version of the distillation you might have used to purify water. If we heat water, it eventually turns into steam, which we can then collect, cool, and condense back to water; that’s distillation, and it produces highly purified or “distilled” water. We can heat and distill crude oil the same way, but all those many hydrocarbons it contains have molecules that are different sizes and weights, so they boil off and condense at different temperatures. Collecting and distilling the different parts of crude oil at different temperatures gives us a bunch of simpler mixtures of hydrocarbons, called fractions, which we can then use for making different types of plastics.

Hydrocarbons made in this way are the raw materials for polymerization, the name we give to the chemical reactions that make polymers. Some polymers are made simply by fastening hydrocarbon monomers together, like daisy chains, which is a process called addition polymerization. Others are made by joining together two small hydrocarbon chains and removing a water molecule (two hydrogen atoms and one oxygen), making a bigger hydrocarbon chain in a process known as condensation polymerization. The more often you repeat this, the longer the polymer gets.
Typically, we need to use other chemicals called catalysts to kick-start polymerization. Catalysts are simply substances we can add that make a chemical reaction more likely to happen and, though they may change temporarily during the reaction, they re-emerge at the end in their original form; in other words, they’re not permanently changed as the reaction takes place. Ziegler-Natta catalysts, some of the most important for making polymers, were developed through the work of German chemist Karl Ziegler and Italian Giulio Natta, which won them a joint Nobel Prize in Chemistry in 1963.
Because we need plastics to do all sorts of things, we often have to add other ingredients to the basic hydrocarbons to produce a polymer with exactly the right chemical and physical properties. These extra ingredients include colorants (which, as the name suggests, turn plastics into all kinds of bright and happy colors), plasticizers (which make plastics more flexible, viscous, and easier to shape), stabilizers (to stop our plastics breaking apart in sunlight and heat), and fillers (typically low-cost minerals that mean we need less of the expensive, oil-based hydrocarbons to make our final plastic product—so we can make and sell it more cheaply).

The plastic-making process doesn’t end there. What we’ve got at this point is a plastic polymer known as a resin, which can be used for making all kinds of plastic products. Resins are supplied as powders or grains that are loaded into a machine, heated, and then shaped by one or more processes to make our finished plastic product. The shaping processes include injection and blow molding (where we squirt hot plastic through a nozzle into a mold to make things like plastic bottles), calendering (squashing between heavy rollers, for example, to make plastic sheets or films), extruding (squeezing plastic through a nozzle, perhaps to make pipes or straws), and forcing plastics through a kind of microscopically small sieve, called a spinneret, to make thin fibers (which is how fibers are made for things like toothbrushes or nylon stockings). There are many other plastic-making processes as well.
What are plastics like?
The many kinds of plastics all have different properties (if they didn’t, we wouldn’t need so many of them in the first place). Having said that, they do have things in common. Generally, plastics are flexible and easy to shape in a variety of ways (remember, that’s why we call them plastics); easy to make in all different shapes, sizes, and colors; lightweight; electrically insulating; waterproof; and relatively inexpensive. Some of them are meant to be very strong and durable (car bits and prosthetic body parts are examples), while others are designed to fall apart in the environment relatively quickly (biodegradable plastic bags, for example). The properties of a plastic can also be deliberately engineered. Suppose we want plastics to be resistant to static electricity so they don’t pick up so much dust; then we can use anti-static additives during the manufacturing process to make them slightly electrically conducting.
What do we use plastics for?

In the early 20th century, plastics were quite a novelty; there were only a handful of plastics and very few uses. Zoom the clock forward 100 years and it’s hard to find things that we don’t use plastics for. Materials science means understanding the properties of different materials so we can use them to best advantage in the world around us. Given what we’ve just learned about the properties of plastics, it comes as no surprise to find them helping us out in building construction, clothing, packaging, transport, and in many other parts of everyday life.
In buildings, you’ll find plastics in things like secondary glazing, roofs, heat insulation and soundproofing, and even in the paints you slap on your walls. There are plastics insulating your electrical cables and carrying water and waste-water in and out of your home. Look around you now and you’ll see plastics everywhere, from picture frames and lamp shades to the clothes on your back and the shoes on your feet. How do all these things get into your life? Up to a third of all the plastic we use finds its way into the packaging we use to protect products (sometimes even plastic products) on the journey from factory to home.

Because plastic means flexible, by definition, we tend to think plastics are relatively weak materials. Yet some are incredibly strong and long-lasting. If you have a rotten wooden door or window, for example, you might chisel out the rot and replace it with epoxy resin filler, a very strong thermosetting plastic that will turn rock hard in a matter of minutes and stay that way for years. Car fenders are now mostly made of plastic—and lightweight car and boat bodies are often made from composites such as fiberglass (glass-reinforced plastic), which are plastics mixed with other materials for added strength. Some plastics are soft or hard as the mood suits them. An amazing plastic called D3O® has an astonishing ability to absorb impacts: normally it’s soft and squishy, but if you hit it very suddenly, it hardens instantly and cushions the blow. (Find out more about it in our article on energy-absorbing materials.)
Plastics and the environment
Most plastics are synthetic, so they’re carefully designed by chemists and laboriously engineered under very artificial conditions. They’d never spontaneously appear in the natural world and they’re still a relatively new technology, so animals and other organisms haven’t really had chance to evolve so they can feed on them or break them down. Since a lot of the plastic items we use are meant to be low-cost and disposable, we create an awful lot of plastic trash. Put these two things together and you get problems like the Great Pacific Garbage Patch, a giant “lake” of floating plastic in the middle of the North Pacific Ocean made from things like waste plastic bottles. How can we solve horrible problems like this? One solution is better public education. If people are aware of the problem, they might think twice about littering the environment or maybe they’ll choose to buy things that use less plastic packaging. Another solution is to recycle more plastic, but that also involves better public education, and it presents practical problems too (the need to sort plastics so they can be recycled effectively without contamination). A third solution is to develop bioplastics and biodegradable plastics that can break down more quickly in the environment.
It’s easy to dismiss plastics as cheap and nasty materials that wreck the planet, but if you look around you, the reality is different. If you want cars, toys, replacement body parts, medical adhesives, paints, computers, water pipes, fiber-optic cables, and a million other things, you’ll need plastics as well. Maybe you think we struggle to live with plastics? Try imagining for a moment how we’d live without them. Plastic is pretty fantastic—we just need to be smarter and more sensible about how we make it, use it, and recycle it when we’re done.
A brief history of plastics
- Ancient people start using plastics (natural materials like rubber, animal horn, and tortoiseshell are made from polymers).
- 1838: Injection molding is developed for diecast metal products (a technology that will later revolutionize plastic-making).
- 1839: Charles Goodyear develops vulcanized (heat and sulfur treated) rubber—an example of a tough, durable cross-linked polymer.
- 1855: Georges Audemars, a Swiss chemist, makes the first synthetic plastic silk fibers using mulberry bark and rubber gum.
- 1856: Alexander Parkes develops the first artificial plastic, Parkesine, by making nitrocellulose from cellulose and nitric acid.
- 1875: Alfred Nobel invents gelignite, a plastic explosive also based on nitrocellulose.
- 1885: George Eastman (of Kodak camera fame) revolutionizes photography by making plastic photographic film from cellulose.
- 1894: Viscose, the first commercially successful artificial silk (a form of rayon), is produced by Charles Cross, Edward Bevan, and Clayton Beadle.
- 1907: Belgian-born chemist Leo Baekeland makes the first fully synthetic thermosetting plastic, Bakelite, from phenol and formaldehyde. He experiments with injection molding around the same time.
- 1920: American John Wesley Hyatt develops the first injection molding machine for plastics.
- 1930: American chemist Wallace Carothers and his team at DuPont accidentally discover a weird new material. It soon becomes nylon, a wildly successful plastic that revolutionizes textile manufacture.
- 1930: Transparent, “Scotch” sticky tape is invented by Richard G. Drew of 3M.
- 1930s: German chemist Eduard Simon accidentally makes polystyrene, initially called styrol oxide and, later, metastyrol.
- 1938: Roy Plunkett of DuPont accidentally discovers PTFE (Teflon).
- 1942: Harry Coover of Eastman Kodak invents plastic superglue (methyl cyanoacrylate).
- 1949: Lycra (a type of polyurethane) is invented by DuPont.
- 1949: American Bill Tritt builds the Glasspar G2, the first production sports car with a body made entirely from fiberglass (a plastic composite).
- 1953: Karl Ziegler develops aluminum catalysts for speeding up polymerization.
- 1954: Giulio Natta develops polypropylene, first made by Italian chemical company, Montecatini.
- 1955: Building on earlier work by Karl Ziegler, Natta perfects Ziegler-Natta catalysts.
- 1954: Dow Corning invents expanded polystyrene.
- 1958: George de Mestral files a patent for VELCRO®, the reusable plastic hook-and-loop fastener.
- 1966: Stephanie Kwolek and Paul Morgan of DuPont are granted a patent for Kevlar®, a super-tough plastic similar to nylon. It’s commercially introduced in 1971. Also in 1966, another DuPont chemist, Wilfred Sweeny, is granted a patent for a chemically similar nylon-relative called Nomex®, a revolutionary fireproof material.
- 1982: The Jarvik 7, a complete artificial heart, made from plastic polyurethane, is first implanted in a human.
- 1988: Australia becomes the first country to issue high-security plastic banknotes.
- 1990s: The first modern 3D-printers are developed. They can make realistic models of objects by squirting out layers of hot ABS (acrylonitrile butadiene styrene) plastic.
- 1997: Captain Charles Moore discovers the Great Pacific Garbage Patch.
- 1998: Smart cars made from composites enter production.
- 2001: Scott White, Nancy Sottos, and collaborators at the University of Illinois at Urbana-Champaign develop remarkable self-healing materials from plastics.
- 2002: British inventor Richard Palmer files a patent for a revolutionary energy-absorbing plastic, which he calls D3O, that can soak up the force from impacts.
- 2016: Japanese scientists report the discovery of bacteria that can eat plastic bottles.
 There are many different plastics, so we need ways of making sense of them all by grouping similar ones together. Here are a few ways we can do that (and there are others I’ve not listed):
There are many different plastics, so we need ways of making sense of them all by grouping similar ones together. Here are a few ways we can do that (and there are others I’ve not listed):







